In just days, this Nutrition Protocol supercharges your Body to destroy Cancer
Main Points
A 5-day, monthly Fasting-Mimicking Diet (low-calorie, low-protein, plant-based) has encouraging human signals when used with standard cancer therapies: it lowers insulin/IGF-1 and can reduce tumor IGF-1 receptor expression, re-primes anti-tumor immunity, and pushes some cancers into an “anti-Warburg” state that slows growth and narrows metabolic escape routes; it isn’t for everyone (e.g., cachexia, frailty, uncontrolled diabetes), and it should be medically supervised, but for appropriate patients it offers a brief, repeatable protocol (Day 1 ≈ 600 kcal; Days 2–5 ≈ 300 kcal; ~10% protein/45% carbs/45% fat; plant-based) that may amplify conventional care and could have preventive potential as evidence matures.
A short, cyclical eating plan called the Fasting-Mimicking Diet (FMD)—five days of low-calorie, low-protein, plant-based meals performed about once per month—has moved from lab curiosity to a protocol with promising signals in people living with cancer. The big questions: does it actually help, how might it work, and what would implementation look like in real life?
What is the FMD?
At its simplest, FMD is a 5-day, plant-based, low-protein, low-calorie protocol. A commonly used template is: Day 1 ≈ 600 kcal; Days 2–5 ≈ 300 kcal/day, with macronutrients around 10% protein, 45% carbohydrate, 45% fat. Cycles are typically repeated every 21–28 days (about monthly), with normal eating between cycles. The goal is to trigger fasting-like cellular programs without a full water fast.
Does it actually fight cancer? (What the studies report)
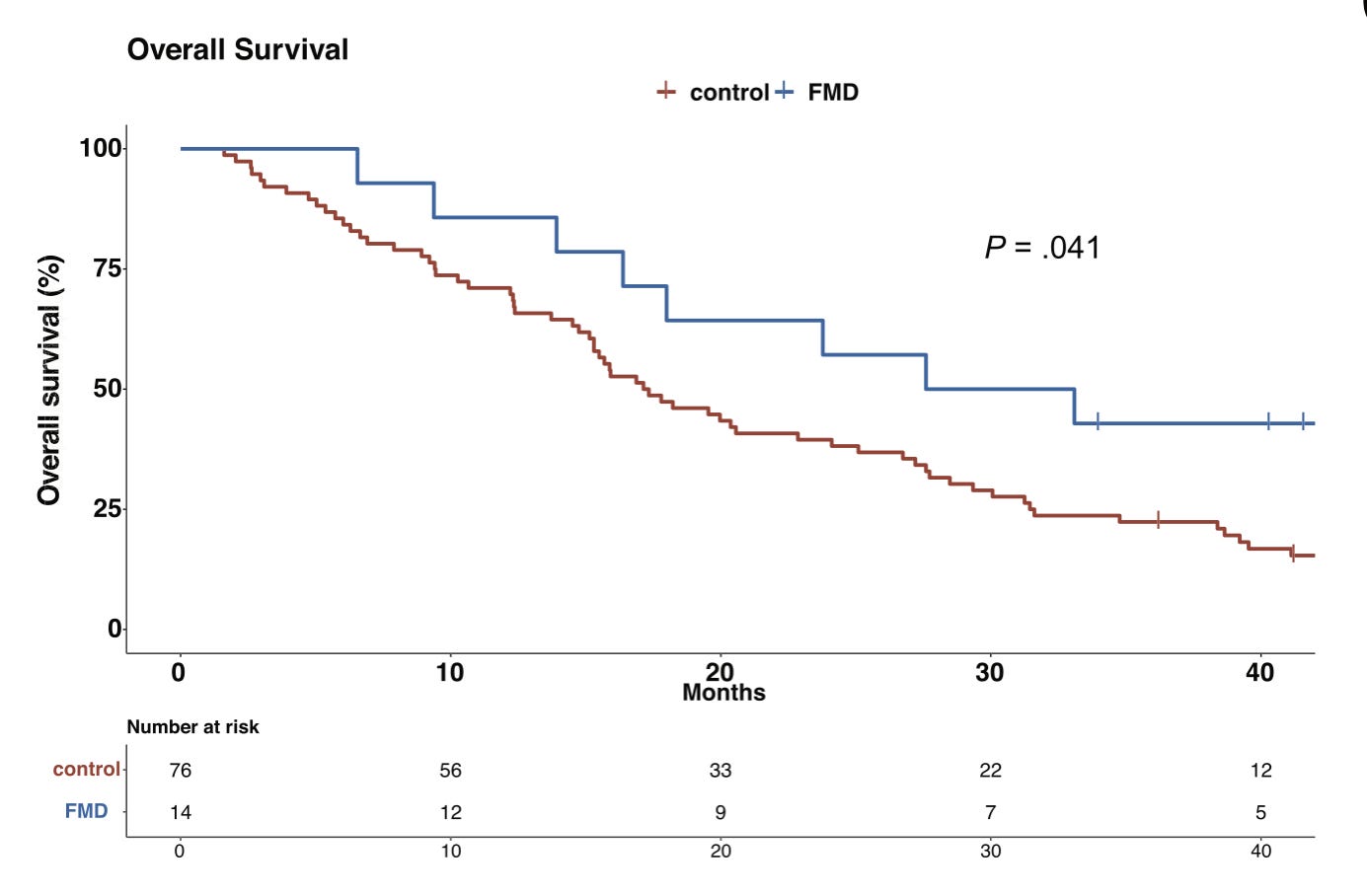
Early human data have progressed to patient studies where FMD was layered alongside standard therapies. In these settings, researchers report better outcomes in FMD groups versus controls, including signals on overall survival in specific cancers (e.g., a sub-analysis in advanced triple-negative breast cancer - see figure below), and favorable shifts in metabolic and immune markers that are relevant to tumor control. Importantly, these trials do not test FMD as a standalone cancer treatment—it is used with conventional care.
How could FMD help?
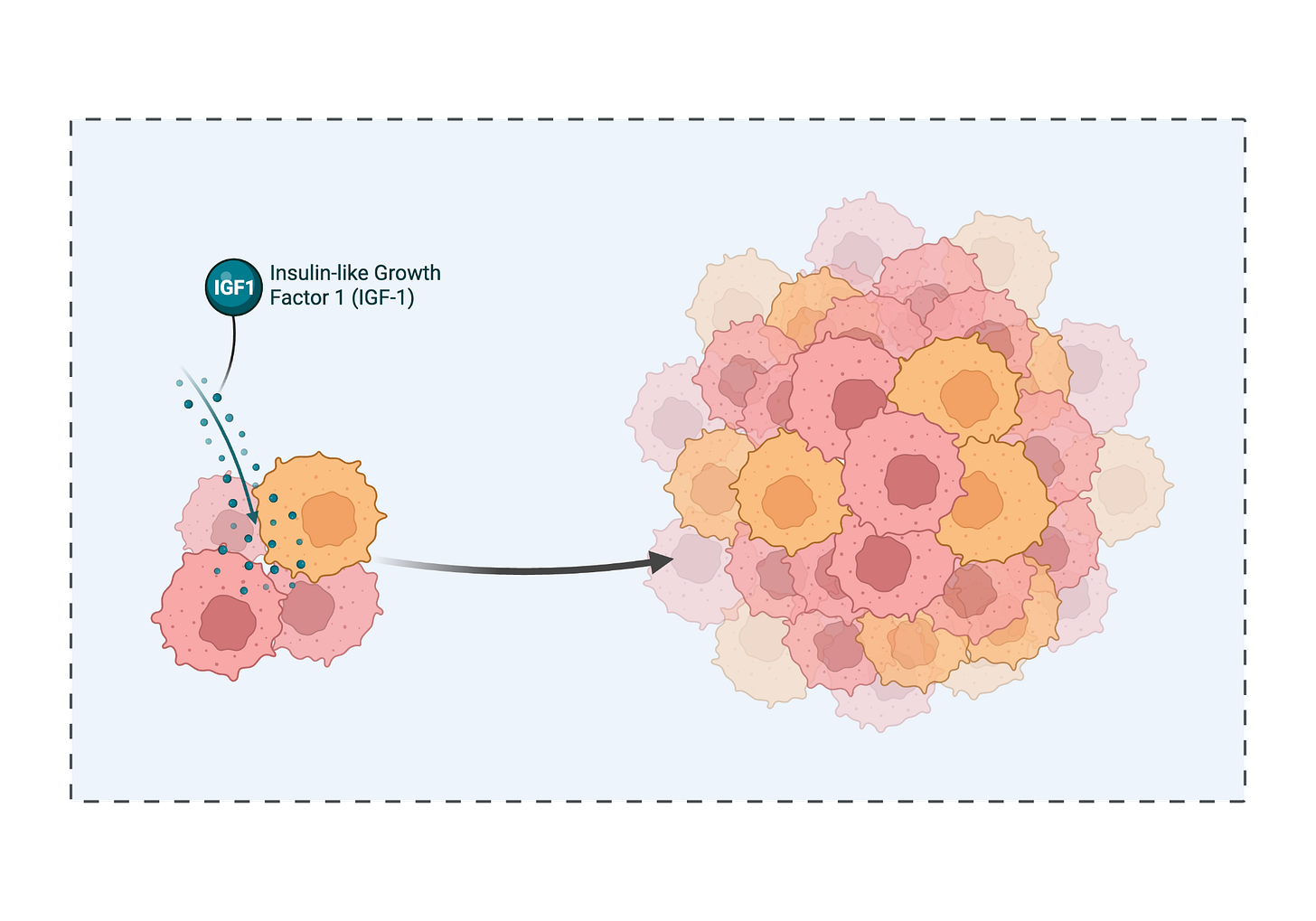
Cuts growth signals: FMD cycles lower insulin and IGF-1 in the blood, potentially blunting a major growth pathway.
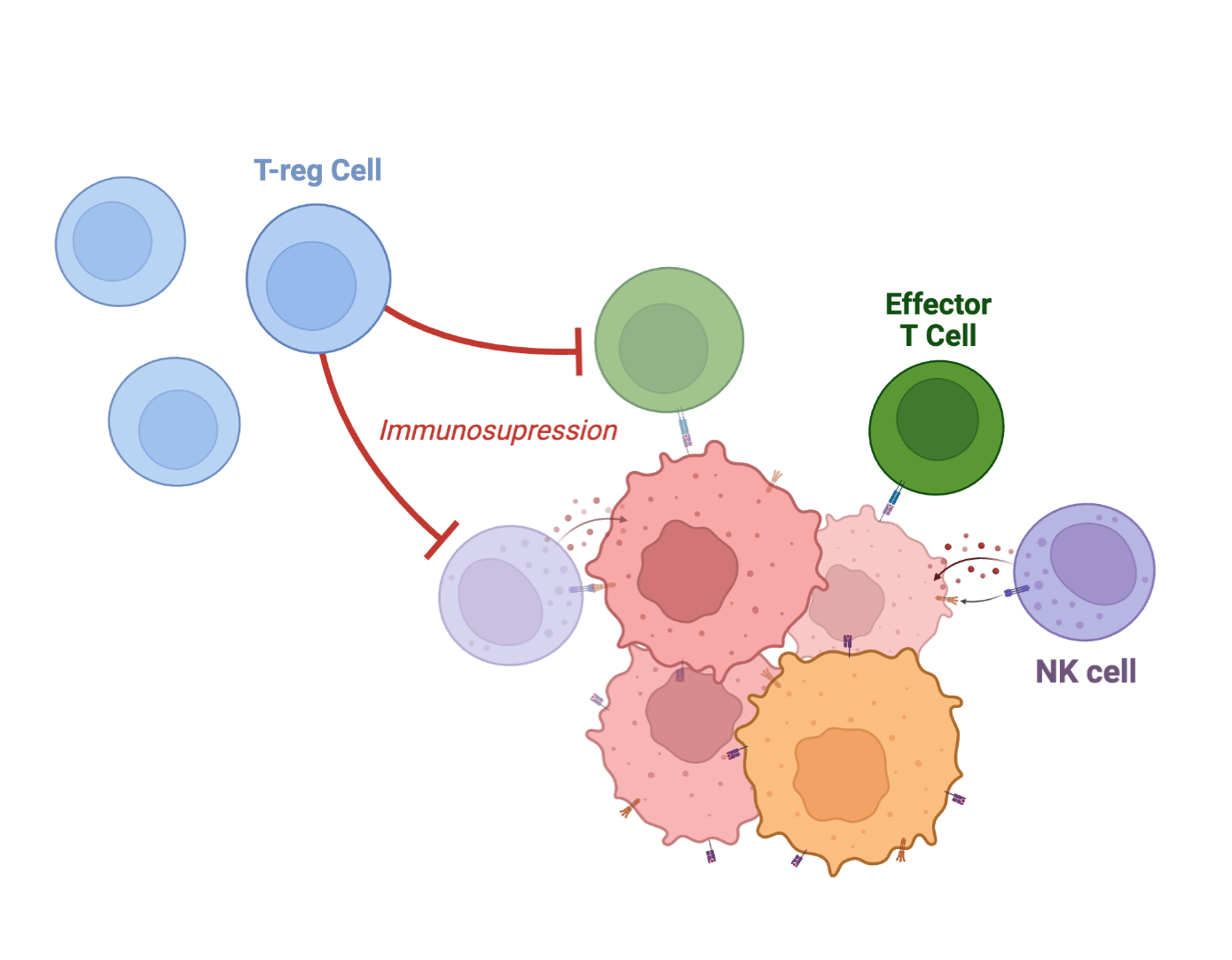
Re-energizes immunity: Studies in patients describe a shift toward anti-tumor immunity—with fewer immunosuppressive cells and more active tumor-fighting cells—tilting the tumor microenvironment against cancer.
“Anti-Warburg” pressure: Many cancers prefer to run on blood sugar using a quick-and-dirty pathway (the Warburg effect). By reducing circulating glucose and calories for a few days, FMD can push some cancers to rely more on mitochondria for energy (less efficient for them), reduce building blocks for rapid growth, and raise oxidative stress inside the cancer cell—together slowing growth and making cells more vulnerable.
A Personalized calculator and plan to achieve more exact FMD numbers is available
More mechanisms and details on how FMD stresses cancer (microbiome effects, ‘Starvation Escape Pathways’)
All of that is included in the complete analysis, along with access to a private podcast, live sessions with me, a library of articles and videos, and much more as a Physionic Insider:
Who is (and isn’t) a good candidate?
Used with oncology care: In clinical studies, FMD was paired with chemotherapy or other standard treatments. Discuss timing and feasibility with your oncology team.
Not for everyone: People with cancer-related weight loss (cachexia), underweight individuals, those with uncontrolled diabetes, pregnancy, eating disorders, or significant frailty should not attempt FMD without close medical supervision—and may be poor candidates altogether.
Prevention vs treatment: Signals suggest FMD may have preventive value too, but the strongest data so far relate to adjunct use in people already receiving treatment.
How to implement (if your clinician agrees)
Plan the cycle: Choose a 5-day window with low work/travel stress.
Hit the targets: Day 1 ≈ 600 kcal; Days 2–5 ≈ 300 kcal/day; 10% protein, 45% carbs, 45% fat; plant-based foods.
Stay monitored: Hydrate, include electrolytes as needed, and track symptoms. People on glucose-lowering or blood-pressure medications may require dose adjustments—coordinate with your care team.
Refeed smartly: Return to balanced eating after Day 5; repeat every 3–4 weeks if tolerated and indicated.
Main Points
A 5-day, monthly Fasting-Mimicking Diet (low-calorie, low-protein, plant-based) has encouraging human signals when used with standard cancer therapies: it lowers insulin/IGF-1 and can reduce tumor IGF-1 receptor expression, re-primes anti-tumor immunity, and pushes some cancers into an “anti-Warburg” state that slows growth and narrows metabolic escape routes; it isn’t for everyone (e.g., cachexia, frailty, uncontrolled diabetes), and it should be medically supervised, but for appropriate patients it offers a brief, repeatable protocol (Day 1 ≈ 600 kcal; Days 2–5 ≈ 300 kcal; ~10% protein/45% carbs/45% fat; plant-based) that may amplify conventional care and could have preventive potential as evidence matures.
A Personalized calculator and plan to achieve more exact FMD numbers is available
More mechanisms and details on how FMD stresses cancer (microbiome effects, ‘Starvation Escape Pathways’)
All of that is included in the complete analysis, along with access to a private podcast, live sessions with me, a library of articles and videos, and much more as a Physionic Insider :
Dr. Nicolas Verhoeven, PhD / Physionic
References
[Study 510] Vernieri C, Fucà G, Ligorio F, et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discovery. 2022;12(1):90–107. doi:10.1158/2159-8290.CD-21-0030.
Funding/Conflicts: Mixed Funding [Public funding: Supported by the Scientific Directorate of Fondazione IRCCS Istituto Nazionale dei Tumori (Milan, Italy); European Union Horizon 2020 (TRANSCAN-2, code TRS-2016-00000393; PI F. de Braud) funded by the Italian Ministry of Health]; Non-profit funding: Associazione Italiana per la Ricerca sul Cancro (AIRC) IG-2017 no. 20752 (PI L. Rivoltini); AIRC MFAG-2019 no. 22977 (PI C. Vernieri).] // Potential Conflicts of Interest [C. Vernieri—grants from AIRC during the study; patents pending PCT/IB2020/055956 and PCT/IT2020/000083; V. Huber—AIRC grant (IG25078) during the study; M.C. Garassino—grants and personal fees from AstraZeneca and Merck, and personal fees from BMS, Roche, Daiichi Sankyo, Celgene, GSK, Eli Lilly, Novartis, Regeneron during the study; G. Bianchi—personal fees from Novartis, Eli Lilly, Roche (outside the submitted work); S. Minucci—patent pending PCT/EP2018/063257; V.D. Longo—non-financial and other support from L-Nutra during the study and other support outside the study; related patents pending; G. Pruneri—personal fees from Roche Foundation One, Bayer, Novartis, Lilly (outside the submitted work); D. Bedognetti—grants from Sidra Medicine during the study; L. Rivoltini—grants from AIRC (IG-2017 no. 20752) and European Community–Horizon 2020 Precious Grant no. 686089 during the study; patent for FMD regimen pending; F. de Braud—patents pending PCT/IB2020/055956 and IT201900009954; relationships with Roche, EMD Serono, NMS Nerviano Medical Science, Sanofi, MSD, Novartis, Incyte, BMS, Menarini Healthcare Research & Pharmacoepidemiology, Merck Group, Pfizer, Servier, Amgen, Incyte; no other disclosures reported by the remaining authors.]
[Study 511] Ligorio F, Lobefaro R, Fucà G, et al. Adding fasting-mimicking diet to first-line carboplatin-based chemotherapy is associated with better overall survival in advanced triple-negative breast cancer patients: A subanalysis of the NCT03340935 trial. Int J Cancer. 2024;154(1):114-123. doi:10.1002/ijc.34701
Funding/Conflicts: Mixed Funding [Public funding: Scientific Directorate of Fondazione IRCCS Istituto Nazionale dei Tumori (Milan, Italy); open-access support provided by BIBLIOSAN.; Non-profit funding: Associazione Italiana per la Ricerca sul Cancro (AIRC; MFAG-2019 no. 22977, PI Claudio Vernieri); Giuliani Foundation—Fondazione Gianmaria e Sabrina Giuliani; philanthropic support from Celeste Ungaro and Maria Annunziata Caforio.] // Potential Conflicts of Interest [Giancarlo Pruneri—consulting fees from Roche, Bayer, AstraZeneca; Filippo de Braud—consulting/advisory for Roche, EMD Serono, NMS Nerviano Medical Science, Sanofi, MSD, Novartis, Incyte, BMS, Menarini, AstraZeneca, Pierre Fabre, Mattioli 1885, McCann Health, Taiho, IQVIA; speaker bureau honoraria from BMS, Healthcare Research & Pharmacoepidemiology, Merck Group, MSD, Pfizer, Servier, Sanofi, Roche, Amgen, Incyte, Dephaforum, Seagen, Nadirex, Ambrosetti, Itanet; research grants/funding from Novartis, F. Hoffmann-La Roche, BMS, Ignyta, Merck Sharp & Dohme, Kymab, Pfizer, Tesaro, MSD, MedImmune, Exelixis, Loxo Oncology, Daiichi Sankyo, Basilea Pharmaceutica, Janssen-Cilag, Merck KGaA; Claudio Vernieri—consulting/advisory for Novartis, Pfizer, Lilly, Daiichi Sankyo; speaker honoraria from Novartis, Lilly, Istituto Gentili, Accademia di Medicina; research grant from Roche; all other authors declare no conflicts of interest.]
[Study 512] Vernieri C, Ligorio F, Tripathy D, Longo VD. Cyclic fasting-mimicking diet in cancer treatment: Preclinical and clinical evidence. Cell Metab. 2024;36(8):1644-1667. doi:10.1016/j.cmet.2024.06.014
Funding/Conflicts: Mixed Funding [Public funding: European Research Council (ERC) grant METABALANCANCER—101117893; National Institutes of Health/National Institute on Aging (NIH/NIA) P01 AG034906; U.S. Department of Defense Breast Cancer Research Program grant BC161452.; Non-Profit funding: Fondazione AIRC per la Ricerca sul Cancro (AIRC) MFAG Id. 22977 (to C.V.); AIRC IG 17605 and IG 21820 (to V.D.L.); Giuliani Foundation—Fondazione Gianmaria e Sabrina Giuliani. // Potential direct Conflicts of Interest [C.V.—advisor/consultant for Novartis, Eli Lilly, Menarini, Daiichi Sankyo, Pfizer; honoraria from Eli Lilly, Istituto Gentili, Novartis, Accademia di Medicina; patents related to the topic; research grants from Roche (to the Institution); D.T.—advisor/consultant for Novartis, Pfizer, GlaxoSmithKline, Genomic Health, AstraZeneca, OncoPep, Sermonix, Personalis, Ambrx, Roche, Gilead Sciences; research grants from Novartis, Popyphor, Pfizer, and Ambrx (to the Institution); V.D.L.—equity interests in L-Nutra and patents related to the topic.]